Overview
The US Food and Drug Administration (FDA) mandates direct use of eSource for clinical research in regulatory submissions. In September 2013, FDA issued its final guideline for the industry on electronic source data in clinical investigations, followed by the 2015 Federal Register (FR) Notice. Consequently, an industry webinar was held to review the final regulation and FR Notice.
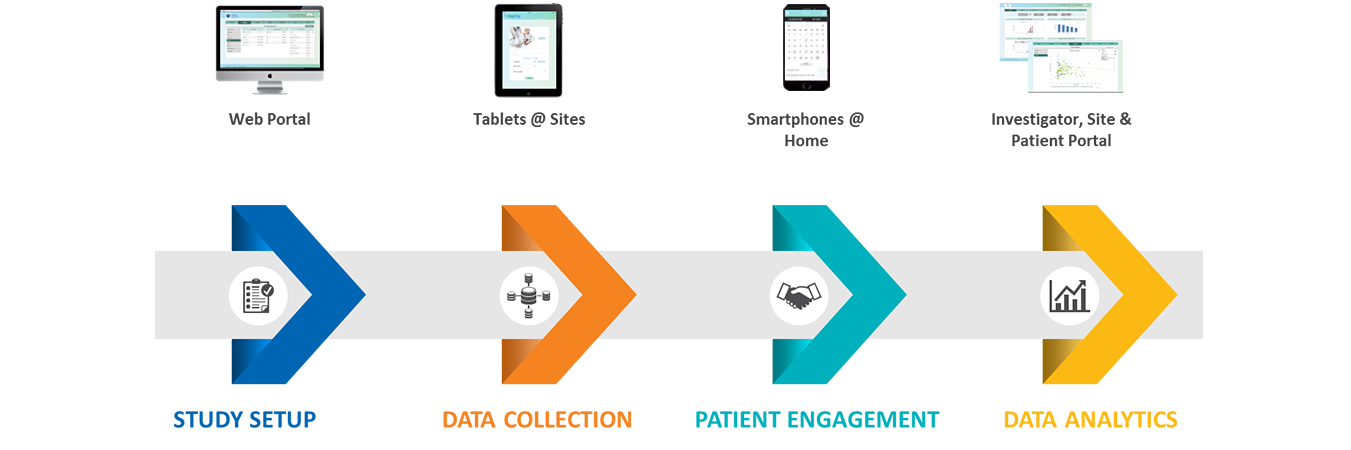
With eSource, we focus on electronic data capture (EDC) efficiency, reducing data queries and monitoring time. With resources being stretched every year, optimizing eSource to achieve clean trial data is now essential for success.
HCLTech Tech optimizes data collection via eSource enabling tablet-based direct data capture and electronic health record (EHR) integration with a fully consolidated clinical trial workflow.
Benefits for Sponsors:
- Improving employee experience for clinical personnel and investigators with minimal discrepancy management, reduced SDV, improved overall data quality and near real-time data access for analysis and reporting
- Boosting patient engagement via eVisits and collaboration portals, ensuring high retention rates and low attrition