Introduction
Integrating Agentic AI into GxP (Good practice) frameworks is reshaping how organizations operate. AI systems now drive efficiency and help teams meet stringent regulatory standards with greater confidence. In this blog, we’ll explore the promise of Agentic AI in GxP use cases, spotlighting how advanced AI can spark innovation, strengthen data integrity and support real-time monitoring.
What is Agentic AI?
Agentic AI brings together the power of large language models (LLMs) with the strategic ability to plan, orchestrate and execute tasks by connecting to a range of tools, data sources and services. Think of it as a team of expert agents working in concert to deliver high-value outcomes. For Agentic AI to deliver its full impact, it must be deeply woven into organizational systems.
Trust in AI is growing, especially in life sciences, where the pressure to accelerate drug development, cut costs, personalize treatments and meet complex regulatory demands is relentless. Traditional workflows are struggling to keep up. Agentic AI steps in to automate multi-step processes, bridge data silos and adapt to evolving tasks and requirements.
Let’s look at a real-world scenario from the life sciences industry where Agentic AI changed the game for validation.
Scenario: Risk assessment for a system change
Background
Risk assessment is rarely straightforward. Typically, a validation subject matter expert (SME) leads the process, working closely with the change owner (business owner) and a mix of stakeholders. It’s not unusual for this to mean lengthy discussions and endless Teams chats just to finalize the questionnaires.
Objective of risk assessment
The aim is clear. Evaluate how changes might affect patient safety, product quality and data efficacy using a set of targeted questionnaires. This process calls for input from several stakeholders, including:
- Business owner
- Functional owner
- Data privacy experts
- GxP and SoX SMEs
How Agentic AI transforms the process
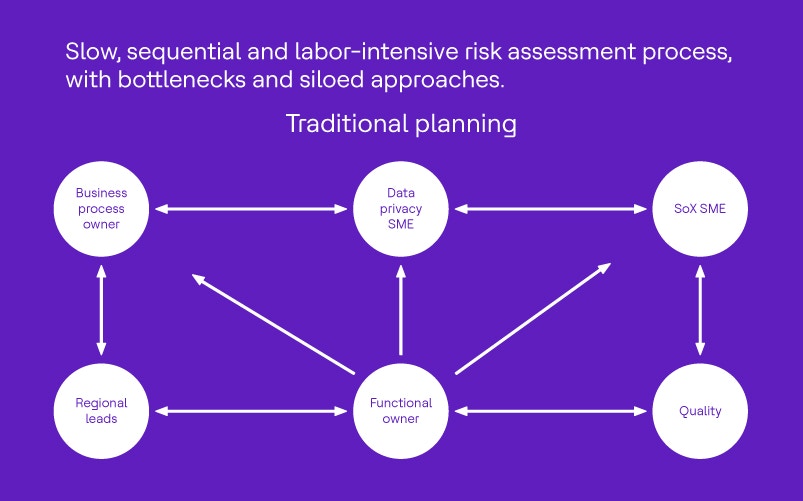
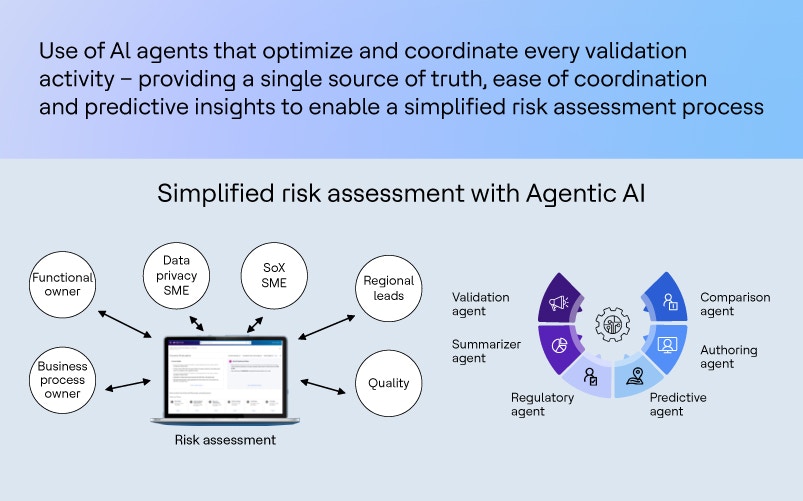
In this scenario, “user” means a business process owner, a functional expert or a quality representative. “Agent” refers to the validation subject matter expert driving the risk assessment.
Workflow
- User input: The user answers the risk assessment questionnaire in natural language
- Agent system workflow: The agent system kicks off a structured workflow, breaking the task into manageable parts and assigning them to specialized subagents, all coordinated centrally
Subagents and their roles
- Regulatory agent: Scans for the latest regulatory updates tied to the input and suggests revisions if needed. Acts as a predictive agent, making sure the input matches current guidelines
- Comparison agent: Reviews conflicting data from different stakeholders
- Summarizer agent: Creates concise summaries tailored for each stakeholder
- Authoring agent: Drafts the risk assessment document for review by a human SME
These subagents come equipped with domain expertise and access to organizational tools and data. They work together, drawing on prior knowledge and best practices to deliver accurate, efficient risk assessments.
Iterative enhancement and impact
The agent system doesn’t stop at a single pass; it refines its output through ongoing interactions. It may ask for more input from stakeholders, such as the business owner, functional lead or quality representative, to keep the output sharp and relevant. The process wraps up with the agent delivering the final output in a pre-approved template that reflects feedback from the review cycle. Once it passes a human-in-the-loop review, the result is formally marked as “approved.”
This approach cuts the typical risk assessment timeline from three to four weeks down to just one to two weeks, saving both effort and time.
Conclusion
Adopting Agentic AI marks a true shift in how work gets done. Unlike hybrid models that lean heavily on human intervention, Agentic AI enables autonomous multi-agent collaboration, managing workflows with minimal oversight. We’re seeing the rise of a digital workforce, where humans and intelligent agents work side by side to deliver results.
For life sciences organizations, this transformation brings both opportunity and challenge; it means embracing higher risk tolerance and adapting to frameworks that may run with limited or no human involvement. Agentic AI offers potential far beyond traditional chatbots or conversational assistants.




