Overview
Software made its entry into the medical field in the 1960s. Further advancements in medical software have been fueled by the rise of electronic medical records and clinical databases.
Recently, several standalone software products (SaMD) have gained popularity and some MedTech companies are focused on integrating software into their existing or new medical devices. In addition to MedTech firms, various IT companies and start-ups are also coming up with unique propositions and targeted solutions aimed at enhancing patient care.
As per the Global IEC 62304/IEC 82304 standard on the software lifecycle processes of medical device software, Software as a Medical Device (SaMD) is defined as a software system that has been developed to be incorporated into the medical device being developed or that is intended for use as a medical device. The US FDA defines medical device software as "any software that meets the legal definition of a [medical] device." Additionally, the EU defines the term Medical Device Software (MDSW) as the software intended to be used, alone or in combination, for a purpose as specified in the definition of a medical device in the Medical Device Regulations (MDR). Software as a Medical Device is defined by the International Medical Device Regulators Forum (IMDRF) as "Software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device."
The MedTech industry faced a central Gordian knot with the advent of the new EU MDR 2017 and the obstacle extends to bringing sufficient evidence to every medical device, including software. The 21st century has witnessed the widespread use of smartphones, which has intensified the demand for further innovation. The COVID pandemic was a breakthrough time for actual innovation, leading to increased efficiencies, minimized errors and reduced operational costs.
Challenges
Over the last couple of years, more than 1000 SaMDs have been approved by the FDA, which highlights the demand and innovation in this market. While everyone is urgently innovating, a key challenge remains: the need to provide adequate evidence demonstrating the accuracy and intended performance of the designated software.
With the expanding role of software in the Medtech industry, potential risks such as bias in automation, erroneous outputs with wrong codes, usability issues, operational inefficiencies and more might lead to serious consequences.
Solutions:
There are several types of medical device software and their need for clinical validation is mentioned below:
Model of software | Intended purpose of the software | Claimed clinical benefit of the software | Need for clinical validation |
|---|---|---|---|
Medical device standalone software | Yes | Yes | Yes |
Software influencing a medical device for medical purpose | Yes | Yes | Yes, for the medical device and the software |
Software driving or influencing the use of a medical device | Yes | Yes | Yes, for the medical device and the software |
Impact
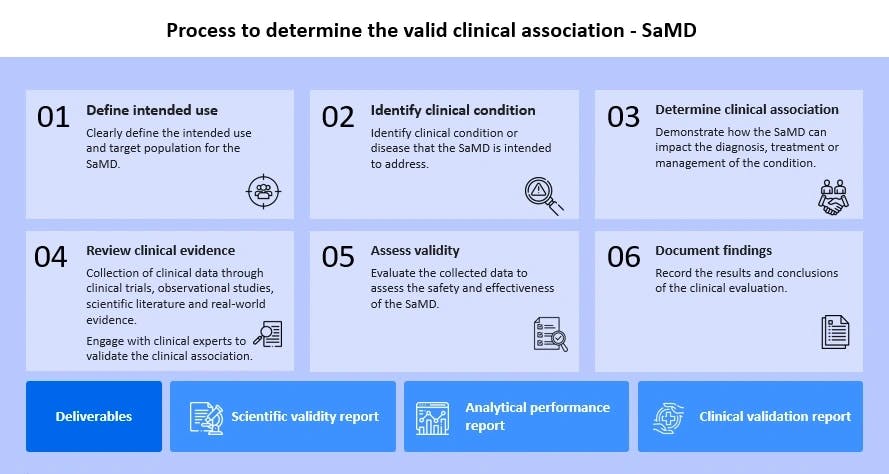
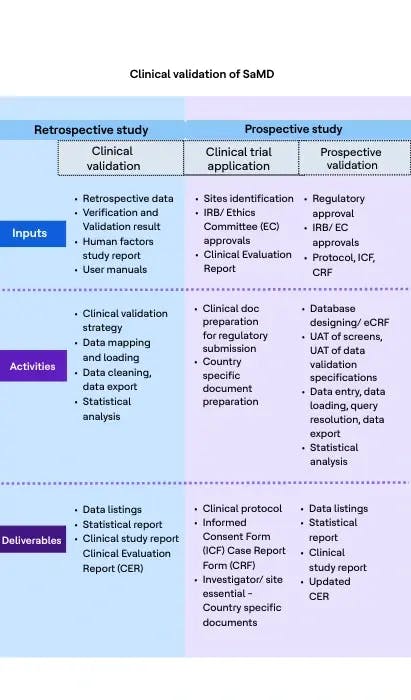
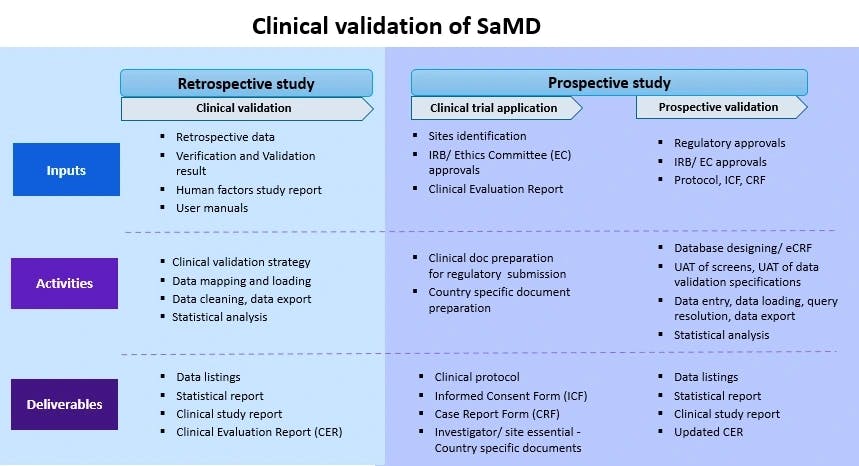
Clinical validation is an integral part of clinical evaluation of a SaMD. It involves a series of ongoing activities that assess and analyze the clinical safety, effectiveness and performance of the SaMD as intended by the manufacturer.
Clinical testing and evidence collection are essential to demonstrate that:
- The software performs as per the claims
- It provides reliable results
- It meets the regulatory standards for patient care
The key aspects include:

The path forward
While companies are on a spree to bring a plethora of SaMDs, it is essential to consider the risk classification of such software based on its intended use for diagnosing, treating or informing patients about their clinical conditions – whether those conditions are critical, serious or non-serious. Hence, it becomes imperative to ensure that the software adheres to the same standards as medical devices by gathering sufficient clinical evidence and demonstrating its performance throughout its lifecycle.


