Executive Summary
Adverse events (AEs) in healthcare aren’t just compliance issues anymore—they’re business-critical. They influence how quickly therapies reach patients, affect brand trust and determine regulatory standing. But most traditional systems weren’t built to handle the complexity, speed or scale of today’s clinical environments. That’s where HCLTech comes in. By integrating advanced AI with platforms like Salesforce, Google Vertex AI and NVIDIA, we’re helping Life Sciences companies evolve into Autonomous Enterprises—intelligent, responsive and built for real-time decision-making.
Why traditional AE detection is failing
The Pharma and MedTech world are undergoing a tectonic shift:
- 80% of new drug launches now focus on targeted therapies, biologics and cell and gene therapies, where AEs are rarer, more complex and harder to detect
- More than 50% of global AE volume now originates from non-traditional sources—call center transcripts, wearable devices, EHR notes and digital health platforms
- Regulatory agencies are increasingly demanding real-time signal detection, proactive safety narratives and evidence-based response timelines
Despite these shifts, many organizations still rely on static, siloed systems. HCLTech is tackling this challenge by building intelligent AE ecosystems that support clinical development, regulatory compliance and post-market safety.
The systemic gap: Epic's sepsis model as a case study for advance reasoning
A 2025 BMJ Quality & Safety editorial recently examined Epic’s sepsis AI model. The verdict? It failed to deliver accurate predictions across hospitals—due to reliance on centralized, untuned data.
In contrast, local subcohort-trained models performed better—reducing false alarms by 50% and dramatically improving clinical utility.
The takeaway is clear: AI in healthcare must be explainable, context-aware and continuously validated.
The cost of missed adverse events
Life science companies face a hidden drag on their innovation engines. 1% of a new drug’s potential revenue is often lost due to delays, rework or inefficiencies in adverse event reporting and resolution.
- $10M in annual loss potential per blockbuster drug
- Innovation slowdown from inefficient AE reporting
- Small biotech risk derailment due to missed signals
From reactive to Agentic AI: A trial team's digital twin in action
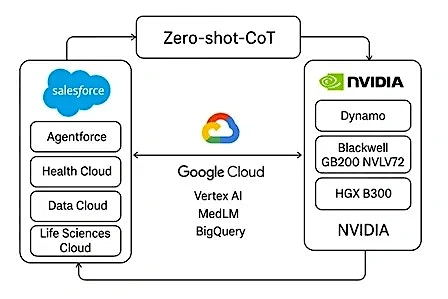
Imagine a multi-agent system that intakes EHR data via Salesforce Life Sciences Cloud, runs Google MedLM for summaries and performs advance reasoning via NVIDIA Dynamo.
For example, during a Phase III trial, a site nurse records a potential AE. Within seconds:
- Agentforce routes the note to an AE agent
- Vertex AI classifies the event as moderate and possibly immune-related
- NVIDIA Dynamo analyzes the patient’s EHR, trial protocol and historical patterns
- A reasoning agent links the event to a rare cytokine response
- A CIOMS-compliant report is generated, documented and reviewed—in near real time
What makes this architecture different:
- HCLTech Agent-2-Agent Salesforce accelerator provides an orchestration layer, in initiating multi-agent workflows for intake, routing, reviewer engagement and regulatory submission
- HCLTech Salesforce Life Sciences Cloud accelerator provides AE-specific objects and invocable actions designed to model, trigger and escalate pharmacovigilance workflows
- HCLTech AE classification with Google Vertex AI, fine-tuned on ontologies like MedDRA, SNOMED and WHODrug, classifies seriousness, expectedness and relatedness with confidence scoring and explainable outputs
- HCLTech AI infrastructure for scaled AI reasoning powered by NVIDIA Dynamo, running on Blackwell, supports inference at scale (50x Hopper throughput) and deep reasoning over 75,000+ token inputs—linking patient context, drug exposure and trial data with auditable logic trails
Mission-critical use cases for reasoning AI:
- Onboarding 10,000 patients across 5 continents in under 6 months
- Triaging hundreds of spontaneous AE reports in oncology drug launch
- Auto-classifying AEs from call center notes with MedDRA alignment
- Auditing AI agent decision logic for clinical protocol compliance
- Tracing regulatory submission reasoning over 75,000-token chains
ROI at a glance: Up to 40% cost savings in AE detection workflows
HCLTech provides outcome modeling where we help clients closely analyze the before and after Agentic AI adoption. The shift to AI-native pharmacovigilance and clinical operations is measurable:
- Fewer false positive escalations
- Faster case closure and HCP notifications
- Reduced burden on safety reviewers
- Real-time safety signal enrichment
| Outcome area | With HCLTech AE AI solution stack | Traditional or DIY Single-Agent approaches |
| Inference cost/patient | ↓ 40% via Dynamo’s 30x throughput | Costly scaling via basic LLMs |
| AE detection time | Seconds | Minutes to days |
| Consent Cycle Time | Instant with agents | Call center delays |
| Trial Onboarding Speed | 3x faster | Fragmented and slow |
| Compliance Logging | Automated & traceable | Manual and error-prone |
| Regulatory Risk | Proactively mitigated | Reactively managed |
Conclusion: Building the autonomous core of enterprise
Perception AI was the first frontier—used for imaging, mammograms and voice recognition. But pharmacovigilance requires more than perception. It requires structured logic, zero-shot inference and domain-aware reasoning. As NVIDIA notes, reasoning inference requires 100x the compute of traditional models and upwards of 100 trillion tokens in training and post-processing to achieve trustworthy results.
LSH enterprises are realizing that the real bottleneck isn’t data, compute, or regulation—it’s reasoning. With HCLTech’s integrated and multi-agent AI architecture, clients can:
- Create AI agents that move from chat to context and compliance
- Enable modular AI fabrics that adapt to clinical workflows
- Transition from SaaS to reasoning-driven decisions
HCLTech’s AE solution offers more than automation. It delivers governance, explainability and value realization across the drug lifecycle. Built with the trusted infrastructure of Salesforce, Google and NVIDIA, it gives LSH leaders the confidence to scale AI in a regulated domain, with outcomes that matter.
Explore what a reasoning-powered safety intelligence ecosystem could look like for your organization. Visit our Salesforce Life Sciences Innovation Hub at - https://hcltech.com/salesforce/agentforce

